Potassium Oxide formula, also known as Potassium Monoxide formula or Monopotassium Monooxide formula is explained in this article. This ionic salt consists of two potassium atoms and one oxygen atom. The chemical or molecular formula of Potassium Oxide is K2O.
It is a pale yellow solid which has no odour. It dissolves in water and is denser than water. It can be obtained by reacting oxygen and potassium. Potassium peroxide is obtained and treated with potassium to produce potassium oxide. It can be synthesized by heating potassium nitrate along with metallic potassium. It is widely used in the Industries as absorbents, intermediates, catalysts. It is generally used in the preparation of chemicals in the chemical industry.
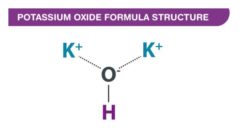
Potassium Oxide Formula Structure
Properties Of Potassium Oxide
| Chemical formula | K2O |
| Molecular weight | 95.204 g/mol |
| Density | 2.3 g/cm3 |
| Decomposes at | 300 °C |
| Melting point | 740 °C |
The contact of this compound with eyes and the skin may severe irritation in the eyes and skin. Inhaling the compound may cause severe irritation in the mucous membrane. It can be toxic when ingested or absorbed by the skin.
To learn more about Potassium Oxide formula from the expert faculties at BYJU’S, register now!
Comments